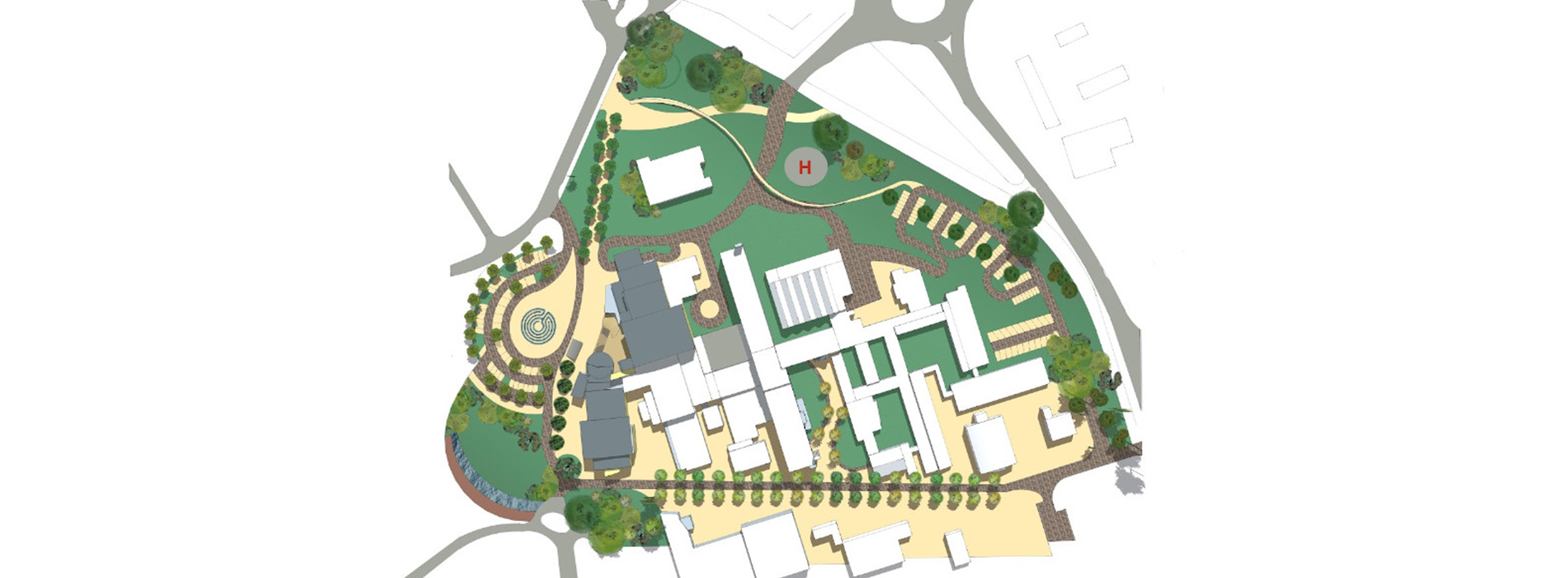
Site Master Plan
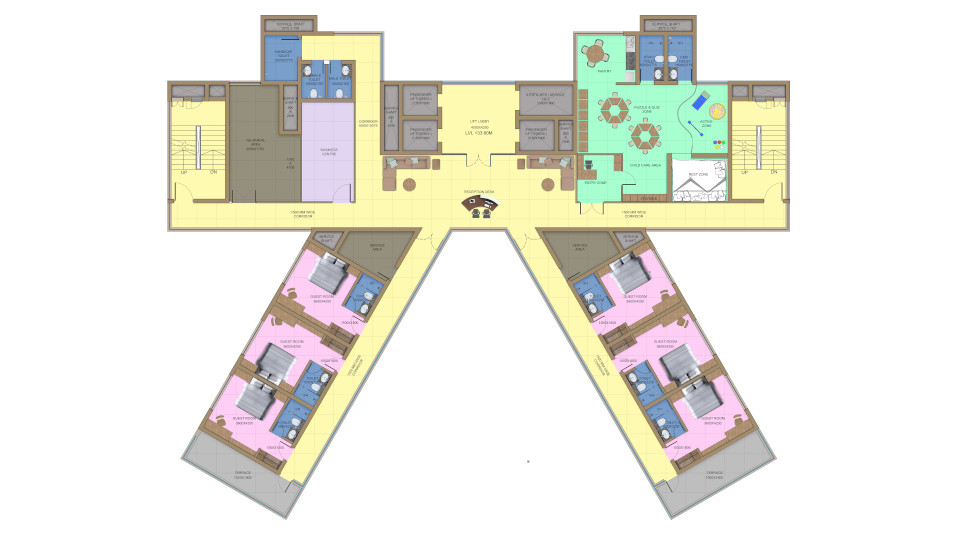
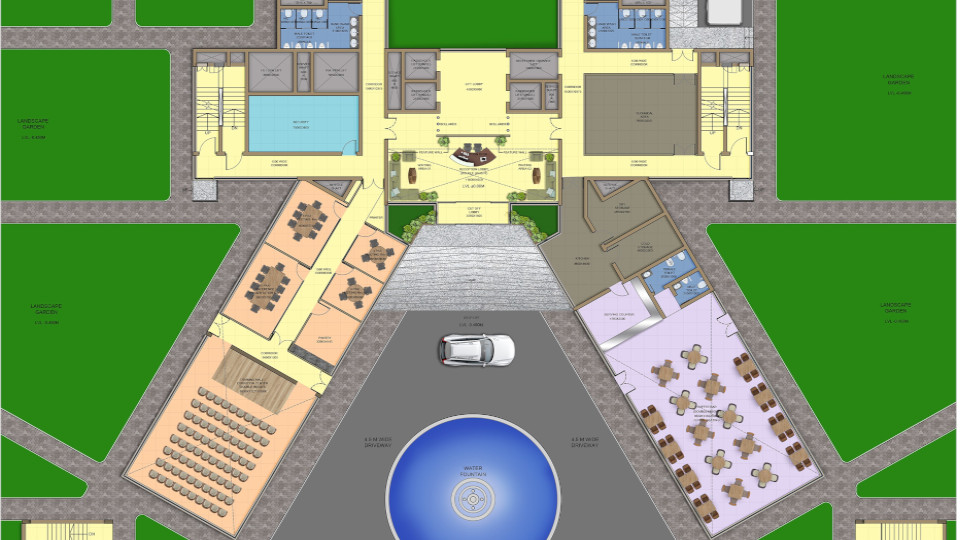
Site Master Plan is the helicopter view of the facility along with all the infrastructure which is planned under the norms of local statutory. Site Master Plan is broadly linked to the overall business strategy of the organisation. The objective of the facility should be well defined and understood. This is an elementary plan that shows overall development based on a Pre – Conceptual or Conceptual Design defining the initial requirements of building spaces, building orientation, flows, connections between buildings, logistics, urban design, infrastructures, and ancillary facilities among others.
It provides a structured approach and creates a clear framework for developing the allocated plot. A key element to determine spaces is to know the usage or application or what type of Drugs are going to be manufactured as according to GMP regulations. As certain drugs must be produced in separate / dedicated space. The master plan looks at the site in its entirety, laying a framework of how individual facilities relate to one another and a phasing schedule for realisation on the site. Preliminary planning will help to derive the tentative size and the floor requirements for all the blocks envisaged in the site. This should cover the preliminary working to estimate all the functional area requirements and distribution of the same in the different blocks with respect to operability, centralized or distributed monitoring and control. The location & orientation of each of the building shall be governed by usage or applications handled within the blocks, operations, direction of wind, activities in nearby plot, topography etc.
Changes are inevitable and are happening everywhere; from global market trends, local customer needs, price of energy, water, raw material and other resources to legislation, risk matters, local matters at the production sites, changes/ modification in GMP guidelines etc. It is needed to clearly allocate every future activity in the given space, so that future enlargements are carried out following a pre-established ideas.
With increasing demand for efficiency and cost reductions it is now more important than ever to optimize the utilization of company assets, such as land, factories and facilities. At the same time companies need to prepare for future changes in demand and legislation. So Site Master Planning must be a holistic approach plan that addresses every issue and yet manages to obtain consensus agreement of management. Typically site master planning will be followed by Conceptual Design of the project.
Each drugs production facility is different & unique however there is common structured approach for the optimum usage of plot.
“Making well-informed and forward thinking decisions”
Typically the deliverables are as under :
- Establishment client expectation and goal
- Assessment of drug production requirement, capacities, constrains, special considerations
- Mapping of the resources available & flow to site
- Future expansion based on business strategy
- Evolution of various possible locations
- Analysis of the application of local Statutory guidelines
- Budget estimate of capital investment
- Analysis & Audit
- Brainstorm session