Life Sciences
Elomatic always thrives to live with purpose of “Idea to Commercialisation”. Elomatic offers end to end services as single window solution provider to client. Elomatic’s holistic approach towards Life Sciences projects encompasses world – class Knowledge, Strategy, Planning, Design, Engineering & Qualification services with seamless integration of multidisciplinary skills.


We, at Elomatic are always driven for “Engineering the Affordable Healthcare” with blend of intelligent engineering. Elomatic is an experienced European consultancy & engineering services provider specialized in various segment of industry inclusive of Global Leadership in Life Science segment Viz. Pharmaceuticals (Sterile & Non Sterile), Biotech, Vaccines, Biopharmaceuticals, Plasma Fractionation, Gene Therapy, Personalized medicines, Containment Formulations, Containment API, Sterile & Non – Sterile API, Nano technology based API, Complex Injections, Large Volume Parenteral, Medical Devices, Fine Chemicals, R&D & Pilot, QC/ QA Labs, Nutraceuticals API, Nutraceuticals Formulations, Enzymes etc.
We have proven track record of successful completion of high-value projects to create Optimised, Sustainable, Functional and Qualified facilities.


Elomatic has evolved into an unparalleled Center of Excellence with all inhouse specialised services & experienced team of Subject Matter Experts (SME) which can deliver end to end (EPCM) consultancy & engineering services. The entire Design, Engineering, Implementation & Qualification of the project is done under the strict compliance of various regulatory guidelines inclusive of local statutory norms.


Each project is customize which requires a multifaceted set of skills. When we design any of the Pharmaceuticals/ Biotech facility, we ensure that all the regulatory guidelines & quality standards are strictly adhered. The motive is to engineered cutting edge Qualified Life Science facility.
Our talent pool is a mix of engineers, pharmacologist, microbiologist & biotechnologists who can understand the clients process/ production needs starting from chemical synthesis or fermentation to the execution of USFDA, EMEA, PIC/s, ANVISA , EU GMP, TGA, UK MHRA, Schedule M, Canada FDA & other global regulatory compliant facilities as per cGMP & GAMP design.
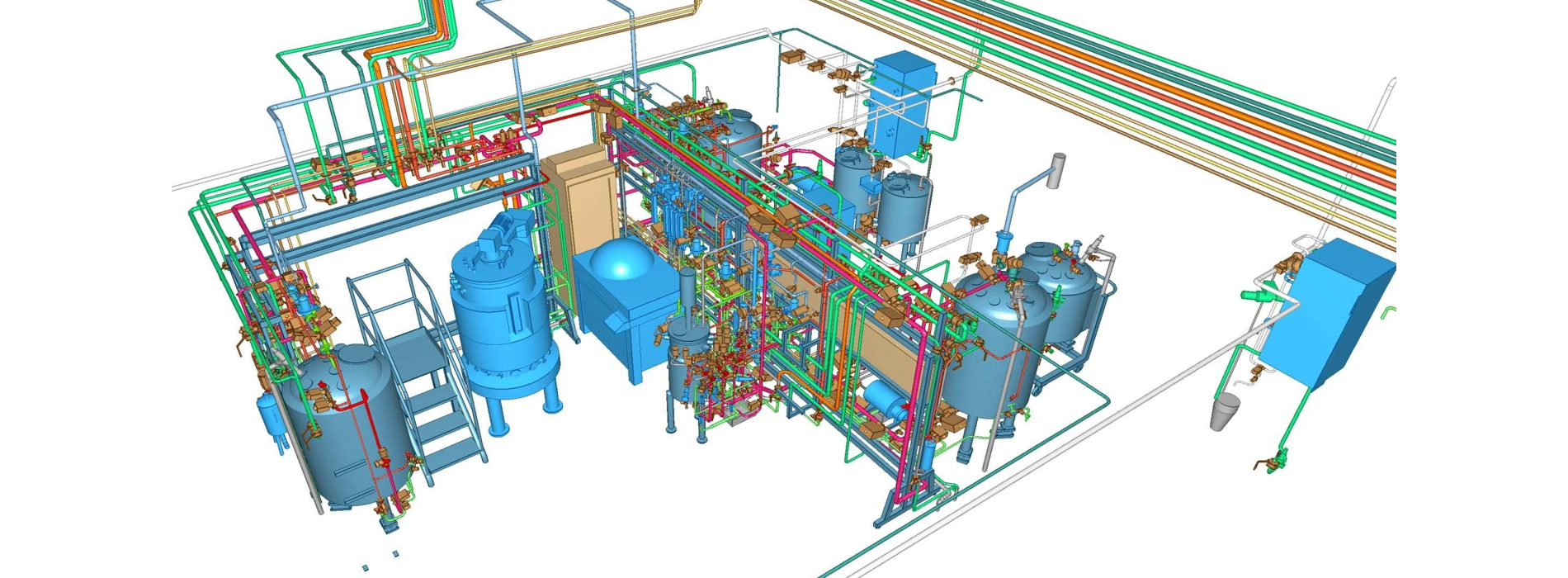
Elomatic offers services starting from a simple OSD project to a complex multiproduct sterile pharma or biologics project. In Biotech, we have the experience of building BSL3 level facility. In addition to compliance, we have expertise in designing high containment & barrier technology facilities.
We design, engineer & build facilities with due consideration to overall facility efficiency, effectiveness & compliance, utilizing domain expertise, cost efficient design, resource/ energy saving parameters, thereby reducing the overall footprint and optimizing CAPEX (Capital Expenditure) & OPEX (Operating Expenditure) without compromising on the Quality of the product and Safety of the human being.
We, at Elomatic get involved at much early stage of projects to evaluate & ensure the complete Value Preposition for the Viability, Sustainability & Constructability of a project. Our philosophy is “Idea to Commercialisation”, complete seamless turnkey consultancy & engineering solutions to Life Science industry.


We have privilege to serve Life Science industry with our valued chain services as listed below :
- Feasibility Report
- Pre Conceptual Report
- Investment Estimate
- Site Master Planning
- Concept Design
- Architecture Design
- Civil & Structure Engineering
- Basic Engineering
- Process Engineering
- Automation Engineering
- Value Engineering
- EHS Engineering
- Office Interior Design with 3D
- Energy Conservation Mechanism
- Waste Management System (inclusive of Bio Kill)
- Procurement Consultancy
- Detailed Design
- GMP Audits & GAP Analysis Report
- Peer Review
- Constructability Review
- Project Management Consultancy (PMC)
- Pre – FAT, FAT & SAT Services
- CQV (Commissioning & Qualification)
- As-built drawings & Documentations
- 3D Modelling (Plant Snapshots & Virtual Walk through)
- Assistance in Technology Transfers & Product Dossier
- Revamping of existing facilities to cGMP compliant facilities
- Training and Knowledge Sharing
At Elomatic, we live with the core philosophy of
Quality, Affordability & Accessibility



